Preclinical Services Program
The Catalyze Program initiatives support product development, including product definition small molecules, biologics, devices, diagnostics and enabling technologies and transformative platforms through five grant funding opportunities.
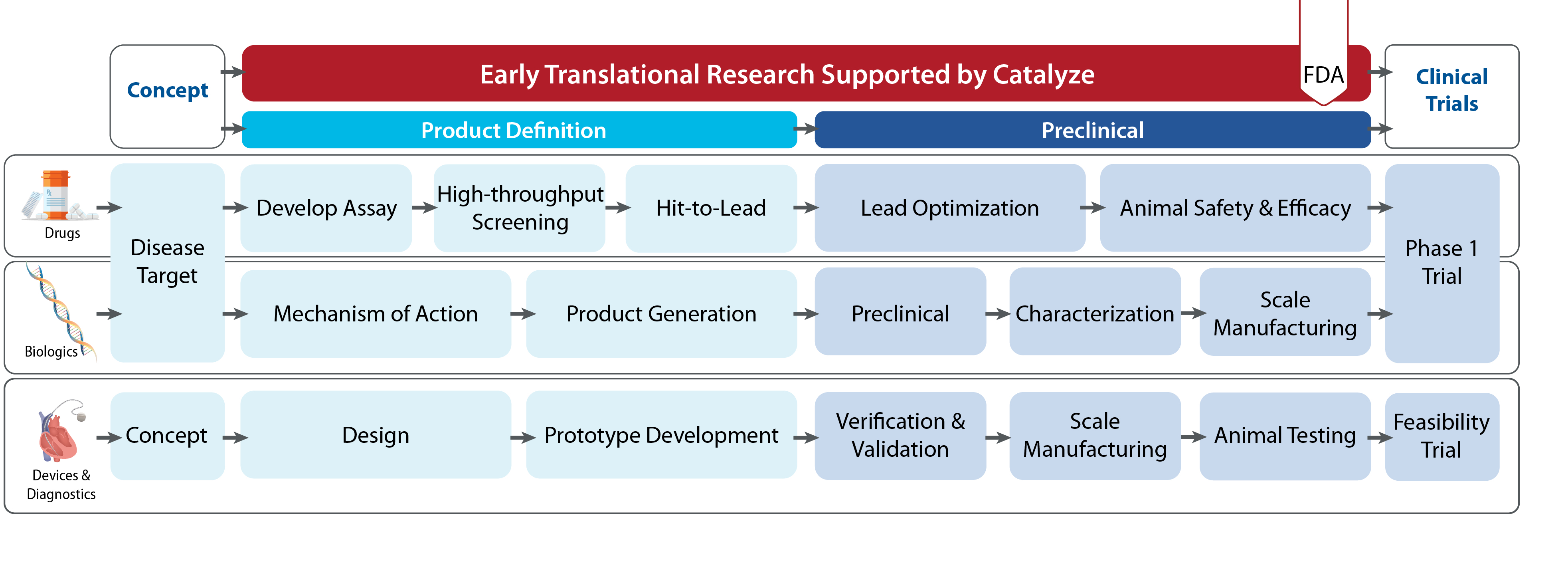
Catalyze preclinical services support preclinical development activities from end-stage proof-of-concept, through preclinical development and US regulatory approval. Applicants request specific services to advance their technology. Applications are sent to external reviewers for evaluation and if a project is selected for support, the level of support and services is negotiated between the PI and NHLBI.
Applicants interested in both preclinical and regulatory services should submit a single preclinical application, which allows an applicant to request both preclinical and regulatory services. Applicants only interested in regulatory services should submit a regulatory only application.
What support is covered?
Preclinical services support can include:
- Animal studies (efficacy, PK, toxicology studies)
- Chemistry, manufacturing and controls (CMC) activities (non-GMP and GMP)
- Device and Diagnostics (prototype development and validation, class determination, scale manufacturing, 510K process)
- Regulatory support (regulatory submissions and FDA meetings)
Informational Webinar
How do I apply?
Step 1
All applicants must submit an expression of interest form (EOI).
The recent EOI submission period ended on February 13, 2026.
Please check back for future application submission periods.
Contact us to request more information
You may download a preview of the EOI form:
Download EOI form
Note: An EOI submission is only needed for preclinical and regulatory services.
Step 2
Selected EOI applicants will be invited to submit a full application.
Application guidelines:
- The application may take 4-6 hours to complete.
- Applications must include Contract Research Organization (CRO)/Contract Manufacturing Organization (CMO) quote or cost estimate to be considered; it is recommended to initiate the process shortly after EOI submission; under current guidelines, the Catalyze Program does not support the use of Chinese CROs or CMOs.
- If you are applying for nonclinical services, you will be required to have a current Certificate of Analysis and Stability Data.
- We encourage you to utilize the PDF copy of the Catalyze Application to gather information needed for submission. You may draft your response and copy/paste into the Catalyze website.
- While the Catalyze program does not enforce a fixed budget cap on applications, in general the program does not support projects above $1,000,000/year, however this can vary depending on product type (e.g., biologics, cell therapy, gene therapy).
- The Catalyze program will not support any GLP/GMP requests before a pre-submission meeting. You may request regulatory support for an FDA meeting.
- Please note that Catalyze Preclinical Services will be provided by CROs/CMOs and managed by RTI International (Catalyze Coordinating Center). The funds will not be given directly to applicants.
- The Catalyze Coordinating Center will contact you by email if there are questions about your application.
- Applicants will be notified of the final decision by email within 4-6 months.
You may download a preview of the application form:
Download application form (PDF)
Download application form (Word)
The referenced Checklists for Preclinical Studies are available below:
Small Molecule
Biologics
Cell and Gene Therapy
Step 3
If approved for Preclinical Services, a requirement of the program is to complete Business Development milestones, which will be negotiated by NHLBI with the PI during the first quarter of preclinical support, and PIs will be expected to complete those milestones. One or more milestones may be required, including, but not limited to the following:
- Engaging with potential partners
- Attending n number of conferences/competing in n pitch events
- Applying to additional funding opportunities
- Discreet goals for end-user engagement (i.e., conduct n interviews across n stakeholder types)
- Performing a competitive analysis and further refining value proposition and competitive advantages
- Developing a business model canvas
- Developing a commercialization plan
- Mapping out funding needed for commercialization
- Market sizing to understand revenue, understanding potential market opportunity
What can I do now to prepare?
- Prepare the key information needed for completion of the application:
- Project plan with key milestones and goals to be achieved at the completion of the project.
- The competitive landscape for the proposed technology and the market size
- The regulatory and reimbursement strategy for your product
- Obtain at least one quote from US-based vendors (CROs, CMOs) within the past 3 months
- CROs/CMOs located outside of the US will need to comply with U.S. State Department regulations as well as NIH policies. Please note, the use of international vendors will require additional clearance from the State Department and may delay the start of Catalyze services.
- Due to the current guidelines, the use of Chinese CROs and CDMOs is not supported by the Catalyze Program.
- Prepare an investor pitch deck
- Identify an Accelerator Partner
- Demonstrate access to non-federal funds or obtain letters of support for non-federal matching funds
Frequently Asked Questions
Please visit the Preclinical section of our FAQ:
Preclinical Services FAQ