Regulatory Affairs Services Program
We are now accepting Regulatory Program Expression of Interest form submissions on a rolling basis.
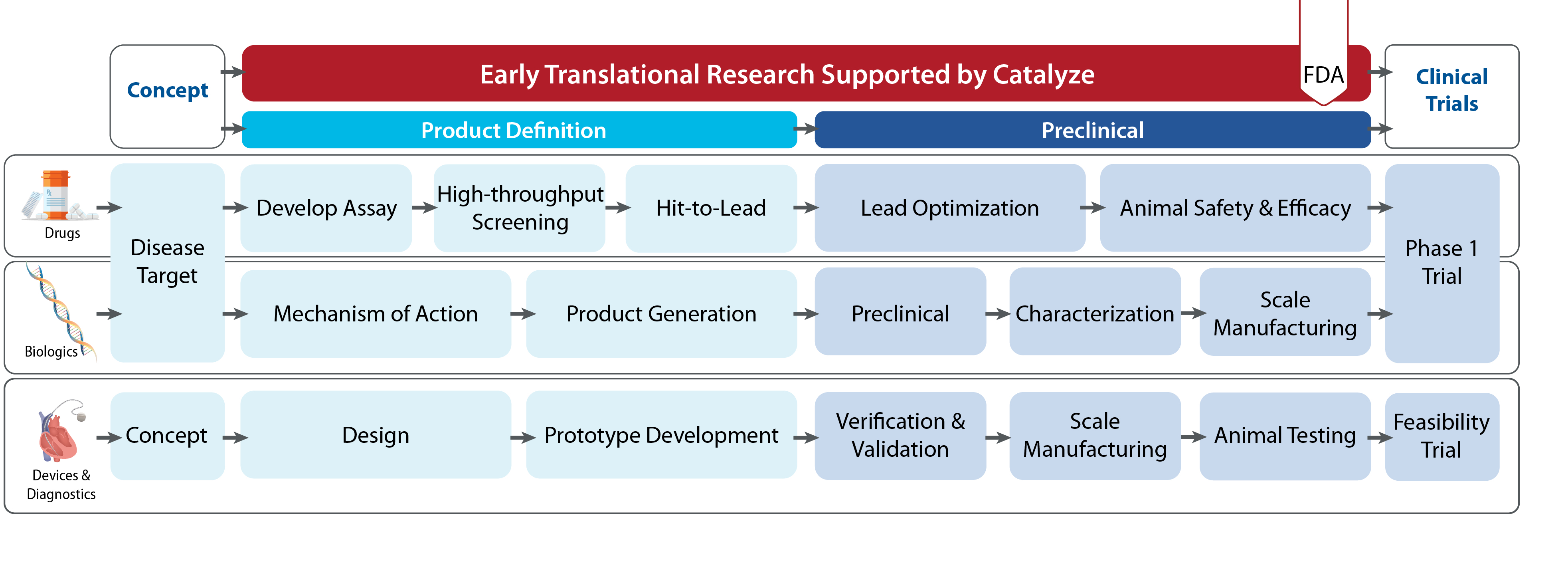
The Catalyze Regulatory Services is a consultation program in which regulatory services are provided through the Catalyze Coordinating Center (RTI).
All applicants must submit an expression of interest form (EOI). Submitted EOIs are reviewed periodically in the order that they are received. Applicants will be notified by email whether they have been approved to proceed to the next step. Applicants moving forward to full application will have 4 weeks to submit.
Applicants interested in both preclinical and regulatory services should submit a single preclinical application, which allows an applicant to request both preclinical and regulatory services.
Since regulatory services are already provided as part of the Catalyze Programs (Product Definition/Preclinical), applicants cannot apply for Regulatory Services if they are already in one of those programs or have a pending Preclinical EOI or Application under review.
What support is covered?
Regulatory services offered by the Catalyze Program include:
- Preclinical development planning
- Developing a regulatory strategy
- Reviewing data and performing a gap analysis
- Assembling documents for FDA meetings (Q-sub, pre-IDE, pre-IND)
- Preparing for a meeting with the FDA
- Preparing an IND/IDE/510k application for the FDA
- Manufacturing development planning
- Quality Management System
- Other
What are the regulatory criteria?
- Applicants cannot apply for Regulatory Services if already in the Catalyze Program (Product Definition/Preclinical) or have a pending Preclinical EOI or application under review.
- Applicant must be a non-Catalyze awardee who meets eligibility criteria, which includes:
- Technology Readiness Level 1-6
- US-based academic, non-profit institutions, and US-owned for-profit institutions
How do I apply?
Step 1
All applicants must submit an expression of interest form (EOI):
Submit expression of interest form (EOI)
You may download a preview of the EOI form:
Download EOI form
Note: An EOI submission is only needed for preclinical and regulatory services.
Step 2
Selected EOI applicants will be invited to submit a full application.
Application guidelines:
- The application may take 3-5 hours to complete.
- We encourage you to utilize the PDF copy of the Catalyze Application to gather information needed for submission. You may draft your response and copy/paste into the Catalyze website.
- The Catalyze Coordinating Center will contact you by email if there are questions about your application.
- Applicants will be notified of the final decision by email within 2 months after the submission of a full application.
You may download a preview of the application form:
Download application form (PDF)
Download application form (Word)